LESSON 1
A Chemistry Deep Dive
The field of Chemistry would consider coffee as a solution, a solute dissolved in a solvent, but chemistry can intimidate with so many unfamiliar terms. Still, when it comes to making coffee, it’s important to understand the chemistry that takes place to produce a good quality coffee – or troubleshoot for flat coffee. So, let’s frame complex chemistry concepts in a familiar way, and dive in.
At its most simplistic, chemistry is just following a recipe using specific ingredients. To avoid flat coffee, then, it’s important to know more about the chemical composition, the ingredients, so that you can maintain optimal flavor.
The Ingredients of Water
Most people know the recipe for water in its purest form, H2O. The ingredients are molecules, the smallest particles that make up matter, the stuff that is all around us. The molecules of water are called Hydrogen and Oxygen, and the recipe follows two hydrogens per each molecule of oxygen. However, there is a twist.
Water is almost never in its purest form; water mixes with all other substances and becomes a solution, a mixture. Whether it’s water in a stream or the water coming from your tap, in addition to the basic ingredients, you’ll find other molecules that will affect the purity of water.
These other molecules in beverage water are not necessarily bad, and in fact, they are familiar to us all as minerals. In chemistry, minerals are called ions, a special type of molecule. These special molecules are found in water as the following common minerals: calcium, magnesium, sodium, potassium, hydrogen carbonate, sulphate, nitrate, and chloride. While not harmful, the presence of these minerals can affect your final product despite the best recipe.
Recipe For Quality Coffee
Like any recipe, the appropriate balance of spices and ingredients will affect the flavor of the final product. The same holds true for brewing good coffee which follows a simple formula of grounds and water. However, it’s anything but simple on the molecular level, a formula that is wise to understand so you can make quality coffee.

Good coffee is flavorful. Flavor is a chemical result of the tongue registering acidity produced by the beans and perceived as ‘sour.’ The right amount of acidity is listed as a point on a number line called the pH Scale that runs from 0, most acidic, to 14, basic (no acidity). If the goal is flavorful coffee, then the chemical solution, water mixed with grounds, should register low on the pH Scale.
While we know the recipe to achieve an appropriate sensory perception of sour, there is a mystery ingredient that can change the final product. There is a mineral found in water capable of altering pH.
The Buffering Effect
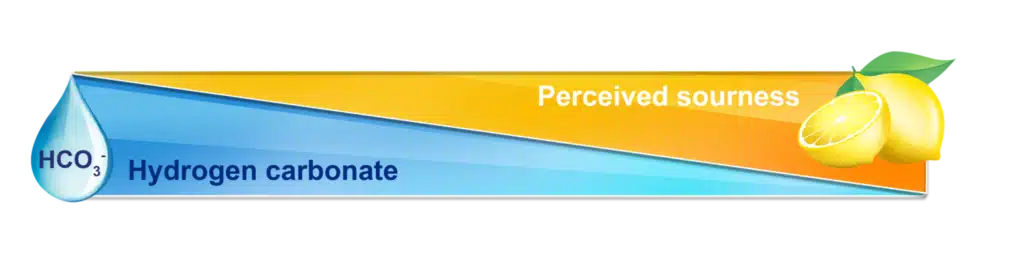
Proportions of a certain mineral, hydrogen carbonate, buffer the perceived sourness flavor by responding to the pH by neutralizing acids. The presence of hydrogen carbonate affects the alkalinity of the water, the ability for water to resist changes in acidity, a key part of the brewing process for coffee! Water must become acidic from the grounds for taste buds to perceive the characteristic sourness of quality coffee.
The buffer capacity of hydrogen carbonate prevents the coffee beans from fully flavouring the water by pushing the pH away from the acidic level required to perceive sour. The water stays too neutral, reducing the flavour to a flat final product.
Solving For Flat Coffee
Like any chemistry problem or troublesome recipe, there are solutions that slightly adjust ingredients to create a better final product. Just as you can add or withhold spices to flavour-to-taste, so can you adjust the water to balance the amounts of minerals within it. The more you can understand these adjustments, the better you can control the outcome, just like any good chemist – or chef.